Autor: Sumithra Urs, PhD | Senior Scientist, Scientific Development and Sylvie Kossodo, PhD | Stellvertretende Direktorin, Wissenschaftliche Entwicklung
Date: December 2021
Most prostate cancers are adenocarcinomas that develop from the gland cells that produce seminal fluid. The American Cancer Society's estimates are about 248.530 new cases in the United States for 2021, resulting in approximately 34.130 deaths.1 About 1 man in 8 will be diagnosed with prostate cancer during his lifetime and 1 in 41 will die of it. 1 Globally, prostate cancer is the second leading cause of cancer death in men, behind lung cancer.1 Fortunately, prostate cancer, when detected and treated early, has a nearly 100 % estimated five-year survival rate for U.S. patients.2 Depending on the tumor stage, treatments can include active surveillance prostatectomy, beam radiation therapy, brachytherapy, hormone therapy, chemotherapy and treatments aimed at bone metastases in Stage IV cancers. To develop novel therapeutics, as monotherapies or in combination with clinical standards of care, testing in the most appropriate preclinical tumor model is key.
Preclinical models that closely reflect human disease are of paramount importance for research discovery and drug testing. There is a limited number of xenograft models that served the field well, especially LNCaP, PC3, and DU145 See here. However, the drawback to using xenograft models is the lack of a fully competent immune system. Syngeneic models, on the other hand, are developed in fully immunocompetent animals, allowing for the testing of immunotherapies and combinatorial approaches. In the preclinical setting, an often-used tumor model is the RM-1 syngeneic prostate cancer model in C57BL/6 mice.3 The tumor cell line was developed from urogenital sinus Zipras/myc9 retrovirus-injected cells of C57BL/6 embryos implanted under the renal capsule of isogenic adult male mice. In the examples shown below, we will show growth characteristics of RM-1 tumors, their immune profile and their response to checkpoint inhibitors and radiation.
In vitro, RM-1 cells grow as an adherent population taking on a fibroblast-like morphology. In vivo, subcutaneous implantation of 5x105 RM-1 cells in the axilla of male 6–7-week-old C57BL/6 mice form aggressive tumors with a median doubling time of ~2 days. The median time to expected staging (100 mm3) is 6 days, and the time to disease progression (2000 mm3) is 12 days on average (Figure 1). Animals show progressive weight gain throughout the duration.
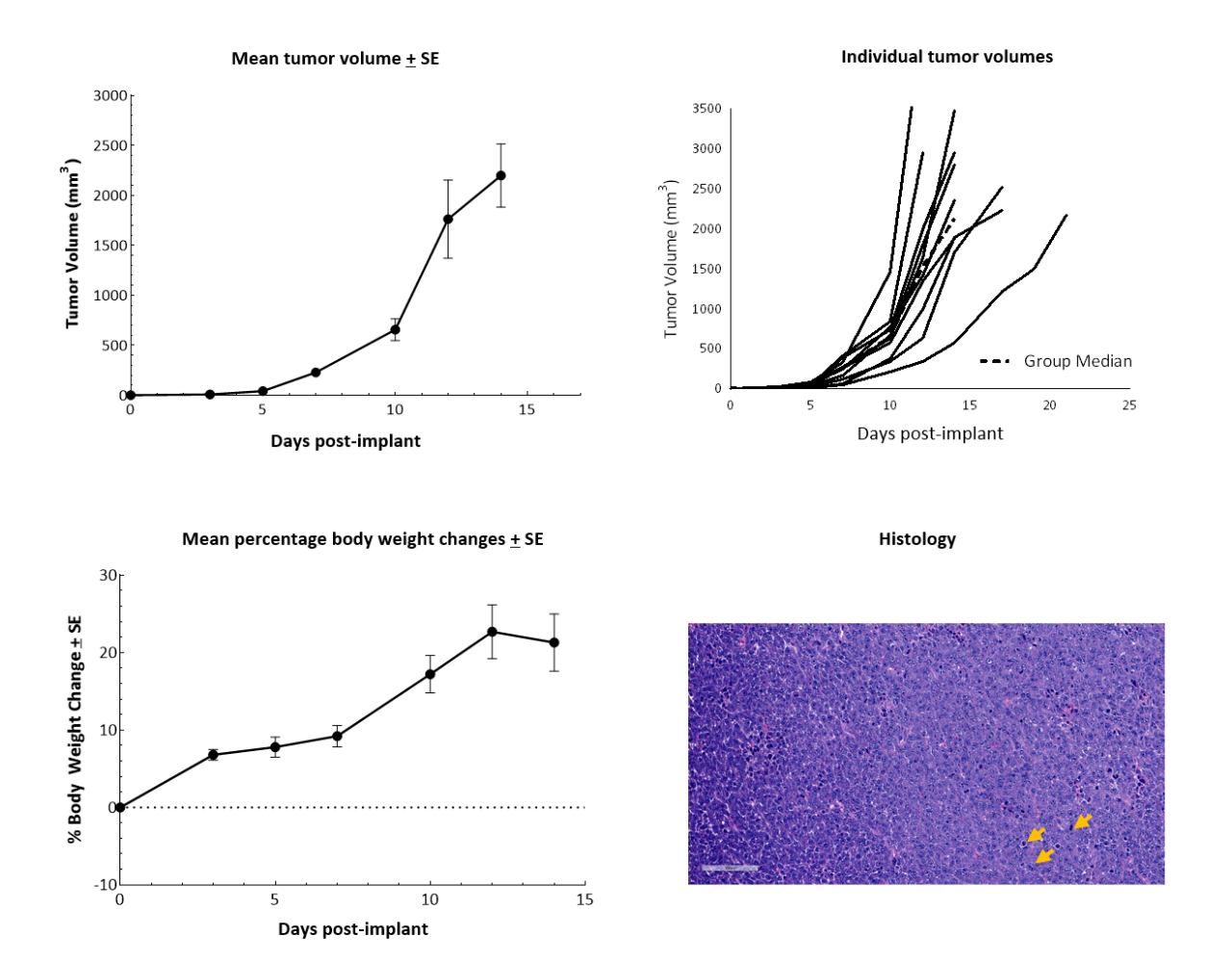
Figure 1. RM-1 cells subcutaneously implanted in the axilla of male C57BL/6 mice. Data based on 9 mice/group. H&E staining of an RM-1 tumor implanted subcutaneously in the axilla of male C57BL/6 mice showing actively dividing cells (arrows).
Immunotherapy with checkpoint inhibitors is currently evaluated in several preclinical studies and Phase 1/2 clinical trials4 for prostate cancer, some of which have shown encouraging results (Phase 2 NEPTUNES trial) using combination immunotherapy with nivolumab (Opdivo®) and ipilimumab (Yervoy®), targeting the PD-1 and CTLA-4 pathways, respectively.5 Likewise, numerous preclinical studies have examined the effect of checkpoint inhibition in mouse models. To understand the anti-tumor efficacy of checkpoint inhibitors against RM-1 tumors in particular, animals harboring subcutaneous tumors were treated with antibodies against murine (m) PD-1, PD-L1, and CTLA-4. Treatment of RM-1 tumors (staged at ~100 mm3) with anti-mPD-1, anti-mPD-L1 or anti-mCTLA-4 only resulted in a partial response in a subset of 20–30 % of the animals (Figure 2). Anti-mCTLA-4 treatment resulted in 28 % increase in time to progression. Overall, therapies did not result in body weight loss (not shown).
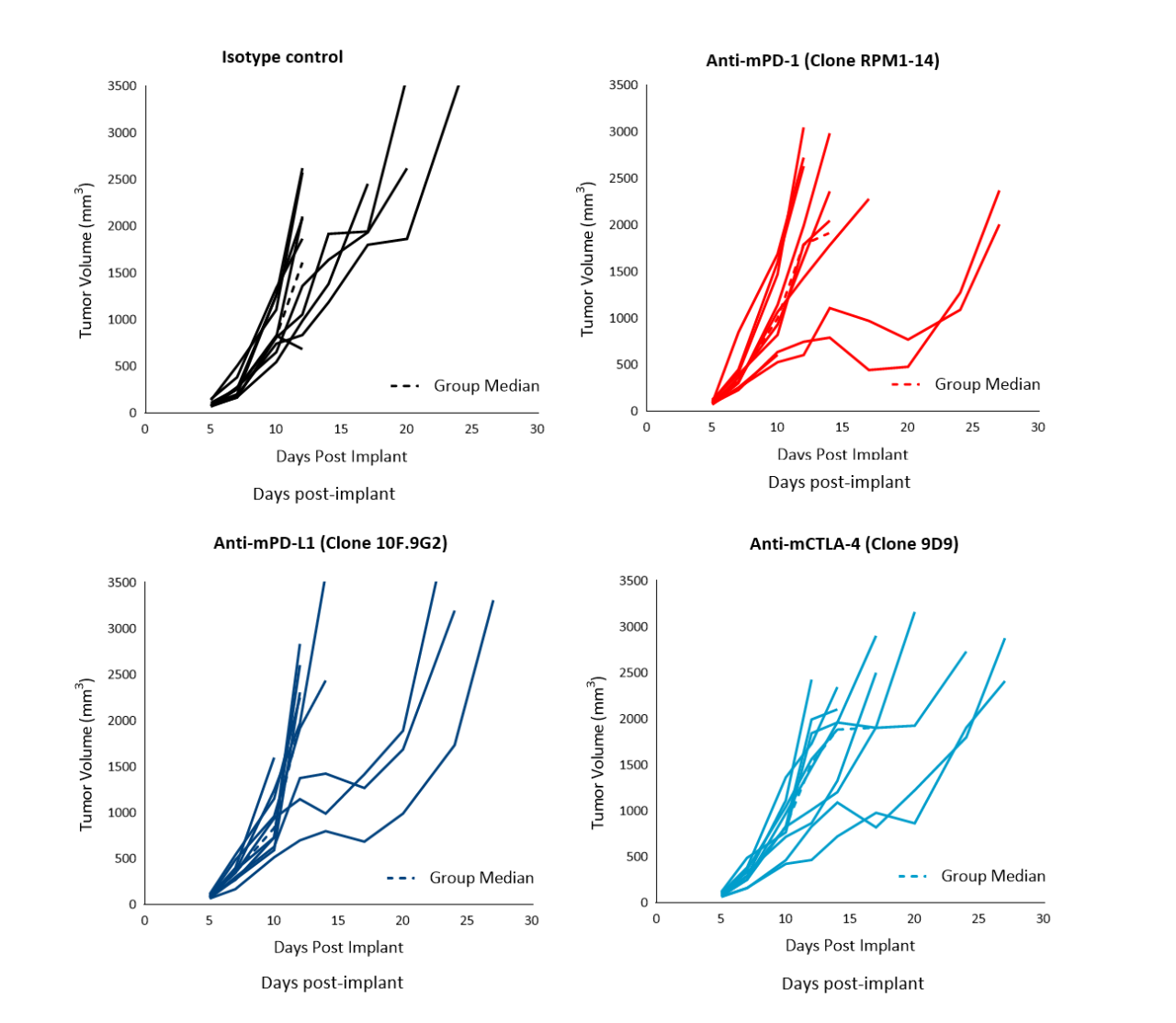
Figure 2. Response of subcutaneous RM-1 tumors following treatment with isotype control, anti-mPD-1, anti-mPD-L1 or anti-mCTLA-4 in male C57BL/6 mice. Data based on 9 mice/group.
To better understand the immune profile of RM-1 tumors, untreated subcutaneous tumors were harvested and dissociated. Cells were then labeled with a comprehensive leukocyte panel (CompLeukocyteTM) and analyzed by an Attune™ NxT flow cytometer. Immune subsets were delineated using FlowJo. Infiltration of lymphoid cells, represented by CD8+ T cells and CD4+ T helper cells, was minimal, suggestive of a “cold” immune landscape (Figure 3). The myeloid population represented a large majority of CD45+ cells and was characterized predominantly by M-MDSCs, G-MDSCs, M1 and M2 TAMs. Taken together, these results indicate that RM-1 tumors exhibit an immune-suppressive milieu in which single checkpoint inhibitor treatment is unable to elicit a significant anti-tumor response.
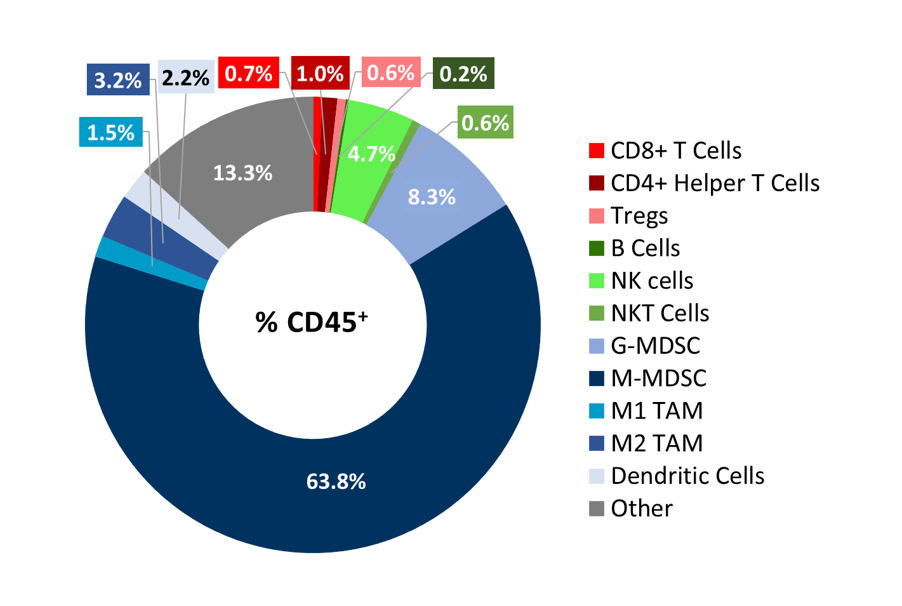
Figure 3. Distribution of immune cell subsets in untreated RM-1 tumors. Tumors (n=6) were collected when they reached ~500 mm3 for flow cytometry analysis with the CompLeukocyteä panel to investigate both the myeloid and lymphoid specific cell populations.
Radiation therapy (RT), with either external beam radiation or brachytherapy (internal beam radiation), is used in patients with prostate cancer at different stages of disease.6 For men with localized prostate cancer, the five-year survival rate using this treatment is 98,8 % overall. We examined the responses of subcutaneous RM-1 tumors to single-dose focal radiation delivered by the small animal radiation research platform (SARRP) from Xstrahl. Single dose of radiation resulted in increase in time to progression at all doses tested: 87 %, 137 % and 350 % at 5, 10 and 20Gy respectively, and 11 % tumor-free survivors and 22 % complete responders at 20Gy (Figure 4). Treatment with focal radiation produced Day 12 median ΔT/ΔC, values of 44 %, 28 % and 6 %, and incidences of putative responders of 88,9 % (8/9), 77,8 % (7/9) and 100 % (9/9), respectively. A lower dose of radiation can be useful in evaluating combination treatment approaches to mimic clinical treatment regimens that include RT.
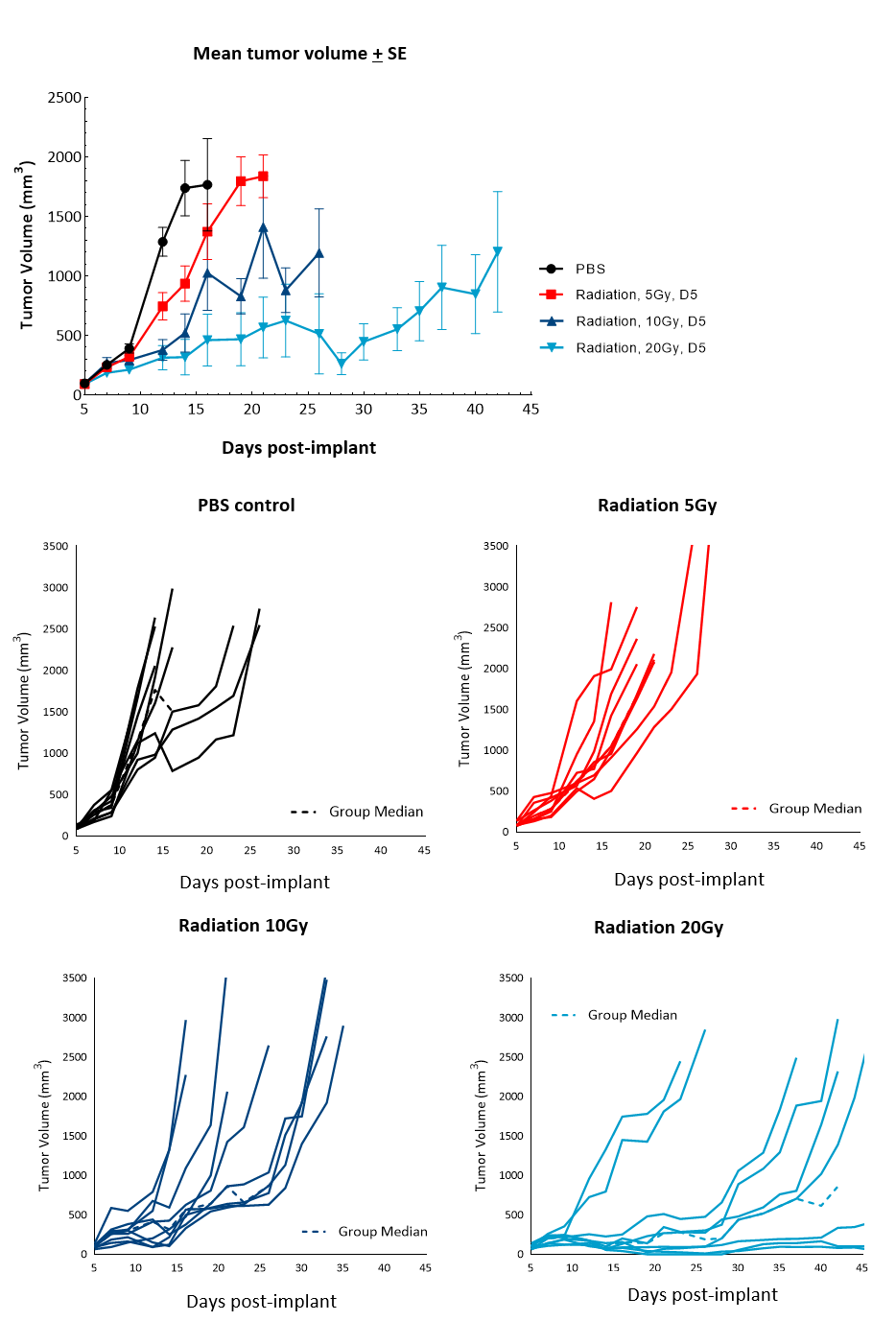
Figure 4. Response of subcutaneous RM-1 tumors to focal radiation in C57BL/6 male mice. Data based on 9 mice/group. Individual tumor growth in PBS-treated control mice or mice treated with focal radiation (5Gy, 10Gy or 20Gy) delivered once.
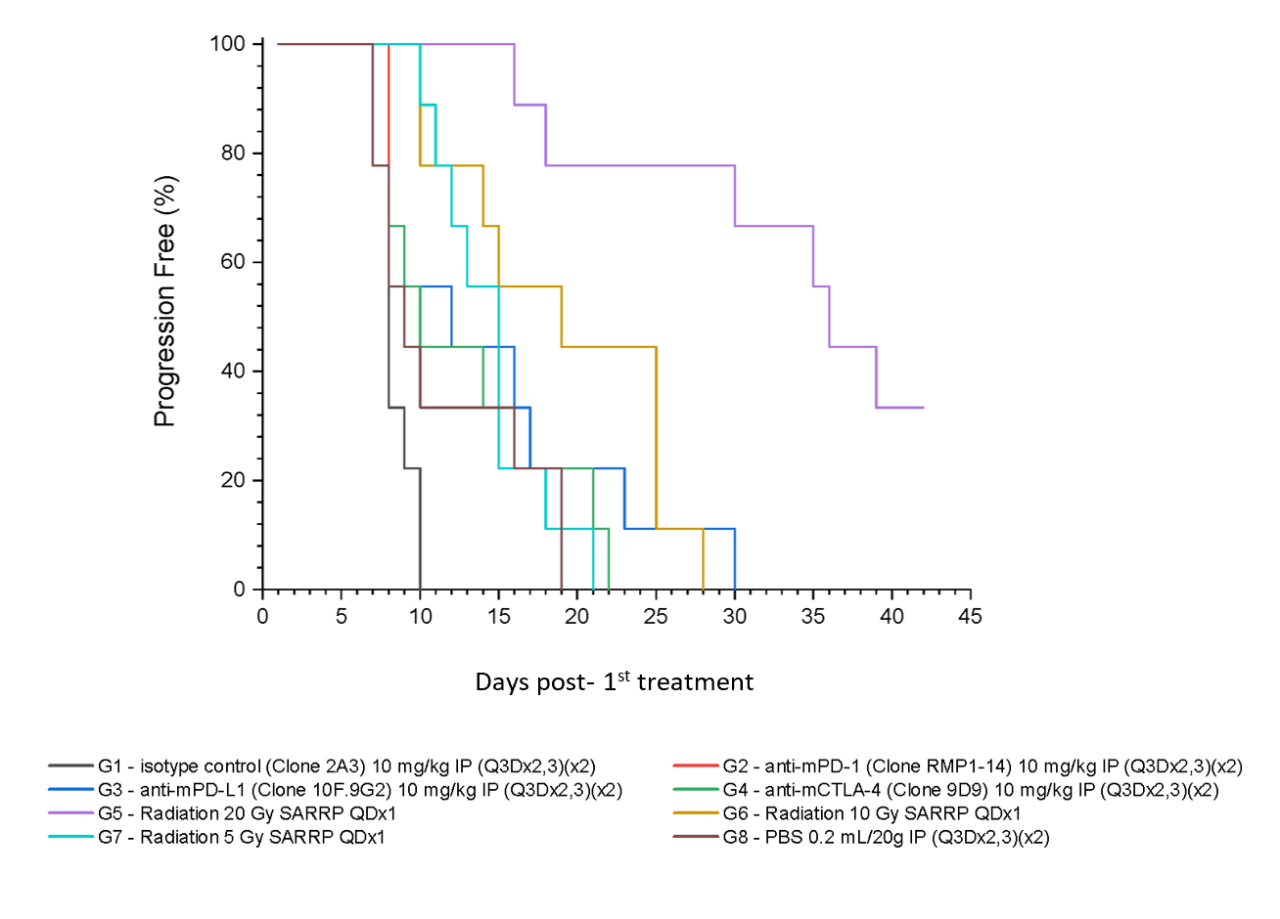
Figure 5 summarizes survival as a function of tumor progression over time. RT showed the best responses, with ~50 % mice still alive after 35 days.
Zusammenfassung
The development of novel therapies relies on preclinical models that closely mimic clinical disease. While syngeneic tumor models do not exactly recapitulate the disease in patients, they do offer advantages over xenograft models. In this Model Spotlight, we review the RM-1 prostate tumor model in syngeneic male C57BL/6 mice. The RM-1 syngeneic prostate model can be employed as a robust preclinical immuno-oncology model in investigating novel treatment combinations with radiation, checkpoint inhibitors, co-stimulatory molecules and/or standard-of-care approaches.
Please contact our preclinical oncology scientists at Labcorp to request the full data set or to learn more about our preclinical oncology services and how they can be applied to your preclinical research.
Verweise
1 https://www.cancer.org/cancer/prostate-cancer/about.html
2 https://www.cancer.org/cancer/prostate-cancer/detection-diagnosis-staging/survival-rates.html
3 Thompson TC, Southgate J, Kitchener G, Land H. Multistage carcinogenesis induced by ras and myc oncogenes in a reconstituted organ. Cell 1989; 56: 917–930.
4 Venkatachalam S, McFarland TR, Agarwal N, Swami U. Immune checkpoint inhibitors in prostate cancer. Cancers (Basel) 2021; 13: 2187.
5 Linch MD, Wong YNS, Jones RJ, et al. Nivolumab (NIVO) and ipilimumab (IPI) treatment in prostate cancer with an immunogenic signature: cohort 1 of the NEPTUNES multi-centre, two-stage biomarker-selected Phase II trial. Presented at: AACR Annual Meeting 2021; April 10-15, 2021; Virtual. Abstract LB004.
6 https://www.cancer.org/cancer/prostate-cancer/treating/radiation-therapy.html
Unterhalten wir uns
Kontakt