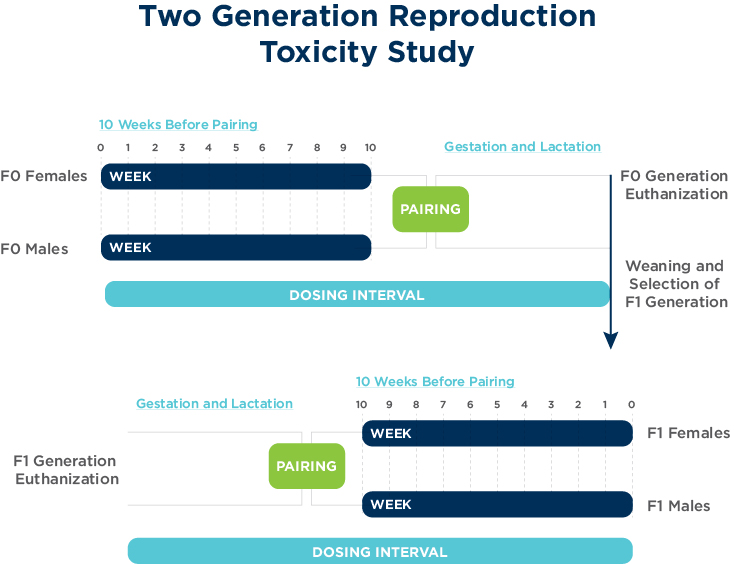
OECD 416: Two-generation reproduction toxicity
The objectives of the two-generation reproductive toxicity study are to detect adverse effects on integrity and performance of the male and female reproductive systems, and on the growth and development of the offspring. The conduct of this study should provide a satisfactory estimation of a no-observed-adverse-effect level (NOAEL) and an understanding of adverse effects on reproduction, parturition, lactation and postnatal development including growth and sexual development.
The OECD 416 study is primarily conducted in rodents via dietary or oral gavage routes of administration.